
How gut health affects resistance to infection
The gut microbiome and the immune system are intricately connected, and over 70 percent of the body’s immune cells reside in the gut. Read on to learn how gut health affects our resistance to infection, and how to best support the gut-immune axis.
A lot sure has changed in the last several weeks. There is no doubt that the global response to the COVID-19 pandemic is affecting everyone, whether in small or large ways. I certainly hope that you and yours are safely social distancing at home and taking the appropriate measures to protect yourselves, your community, and our healthcare workers on the front lines.
It’s taken me a while to decide how I want to tackle the topic publicly. I honestly questioned whether it was even my place to tackle it at all. Many physicians, researchers, and organizations that I follow and trust have put out some really amazing resources in regards to how best we can protect our own health and our broader community against COVID-19. At the same time, I’ve seen a lot of misinformation from people in the online health space about this pandemic. I have no pretenses about being an expert in infectious disease and certainly don’t want to contribute to the noise.
Moreover, as much as what is going on now with the COVID-19 pandemic is and should be on everyone’s mind, we are still in the midst of a long-burning epidemic of gut dysbiosis and chronic disease. And given that approximately 90 percent of patients that need to be hospitalized with COVID-19 have one or more underlying conditions (including obesity, hypertension, chronic lung disease, diabetes, and cardiovascular disease), I still see that area as where my knowledge, energy, and content production efforts are best directed.
Nevertheless, it has always been my mission to empower you with the information you need to support your health and live well, so I decided that I would approach this topic more generally, as this is likely not going to be the only time when it might be important to consider how our gut health impacts immunity.
Thus, this article will be a broad overview of what we know and don’t know about how our gut health influences resistance to infection. I will start with just a few brief notes on the transmission and presentation of COVID-19 that will hopefully provide context for the remainder of the article.
If you’re looking for more detailed evidence-based information about COVID-19, I recommend checking out the videos by MedCram or Ninja Nerd Science and the research updates provided by the New England Journal of Medicine in addition to keeping up to date with WHO, CDC, and local recommendations.
On COVID-19 and the gut
- While the novel coronavirus, SARS-CoV-2, seems to be principally spread by respiratory droplets, the fecal-oral route is also a potential route of transmission.1,2 This means that the live virus may be shed through fecal material and spread to the mouth or other mucosal surfaces through unwashed hands.
- Entry of the virus into the body requires both the cell receptor ACE2 and a transmembrane protein called TMPRSS2.3 These proteins are not only present in the type 2 lung alveolar cells but are also highly expressed in absorptive cells of the small intestine and colon.4
- Many people present with digestive symptoms. According to a recent report, 50.5 percent of individuals admitted to the hospital for COVID-19 in Hubei, China presented with digestive symptoms, including diarrhea, vomiting, and abdominal pain.5 For many patients, digestive symptoms preceded the development of cough, shortness of breath, or fever. A small percentage may present with digestive symptoms alone.
- The virus can be shed in stool, even for as long as five weeks after respiratory symptoms have resolved.6
- COVID-19 has been reported to cause gut inflammation.7 Whether it is capable of producing any long-term damage to the gut, as it seems to be able to do in the lungs, is still unknown.
The gut barrier: first line of defense
To start, I’m going to review the basics of the gut barrier and gut immune system. In many ways, we can think of the interior of the gut as being outside of the body. Much like the hole of a doughnut is not actually part of the doughnut, the gut is really just a hollow tube that connects the mouth and rectum.
The gut barrier is composed of a single layer of specialized epithelial cells that are about the width of a human hair. These epithelial cells must form a tight barrier to prevent microbes, microbial components, and large dietary proteins from entering the body.
To maintain a tight barrier, gut epithelial cells secrete a wide range of antimicrobial peptides, which repel microbes from the epithelium, and a thick, protective layer of mucus that sits on top of the epithelial layer. Protein complexes called tight junctions connect adjacent epithelial cells, preventing anything from slipping through the cracks between epithelial cells.
If the gut mucus layer or gut barrier breaks down, this is called intestinal permeability, or “leaky gut”. This intestinal permeability gives microbes easier access to the body and can lead to chronic inflammation or infection if these microbes overwhelm the gut immune system.
The gut-immune system: 101
An estimated 70 percent of the immune cells in our bodies reside in the gut. These immune cells play a crucial role in determining which microbes are able to colonize and survive in the gut.
Our gut microbes, in turn, are constantly communicating to our gut immune cells. These immune cells lie just beneath the gut barrier in a region called the gut-associated lymphoid tissue, or GALT.

Figure 1. Architecture of the gut-associated lymphoid tissue. Reprinted from Brucklacher-Waldert et al. Cellular plasticity of CD4+ T cells in the intestine. Frontiers in Immunology (2014). https://doi.org/10.3389/fimmu.2014.00488
Early in life, the GALT undergoes a maturation process as the gut immune system learns to distinguish harmless host cells, dietary proteins, and commensal bacteria from pathogenic microbes and environmental toxins. This education of our immune system is essential for its proper functioning. In fact, mice that are raised in a sterile environment (germ-free mice) and do not have a gut microbiota have a severely underdeveloped gut immune system.8
Throughout life, gut microbes continue to shape and regulate the immune system. Gut immune cells have specialized receptors that sense the presence of microbes. This microbe-sensing is critical to maintaining gut homeostasis and gut barrier function.
Alright – now that we’ve reviewed the basics, lets get to the more interesting research!
A healthy gut keeps out potential invaders through colonization resistance
In a recent article, I discussed the concept of nutrient-niches within the gut.
A niche is a multidimensional space of resources and environmental conditions that define where an organism can survive and grow.
In the gut, available niches are primarily determined by the nutrients that comprise the host diet, hence the name nutrient-niches.9
When our gut microbiota is intact, all of the nutrient-niches are completely filled by microbes. This means that any new or harmful microbe will find it difficult to find a niche within which it can colonize and survive in the gut. This is a concept called colonization resistance.10
If our gut undergoes a significant disturbance, however, it will no longer resist invasion by new microbes. Antibiotics, for instance, can “unseat” microbes from their nutrient-niches, allowing new or existing pathogens the opportunity to take hold and cause infection in the gut.
As we’ll see in the next few sections, a poor diet, stress, and other environmental factors can also alter nutrient-niches and reduce our resistance to gut infection.
Dietary fiber supports the gut barrier and reduces susceptibility to infection
When we consume dietary fiber, our gut microbes ferment it into short-chain fatty acids (SCFAs). These SCFAs provide energy for the cells that form the gut barrier, and also stimulate the secretion of a protective layer of mucus that keeps microbes a safe distance from our gut epithelium. The three most abundant short-chain fatty acids in the gut are butyrate, acetate, and propionate.
In 2018, a group of Stanford researchers found that bacteria in the genus Bacteroides produce the SCFA propionate, which limits the growth and colonization of Salmonella.11 Similarly, depletion of butyrate-producing Clostridia has been shown to allow for expansion of Salmonella in the mouse gut.12 Butyrate has also been shown to increase the resistance of intestinal epithelial cells to toxins produced by C. difficile through supporting gut hypoxia.13
Consuming fiber may be essential to producing enough of these SCFAs to effectively repel incoming pathogens. Work done by Dr. Eric Martens’ lab at the University of Michigan found that in mice, depriving the gut of dietary fiber results in a significant expansion of microbes like Akkermansia muciniphila and Bacteroides caccae, which are able to feed on host mucus glycans.14 While these microbes have been shown to be beneficial at low abundance, their rapid expansion and increased activity degraded the protective gut mucus layer.
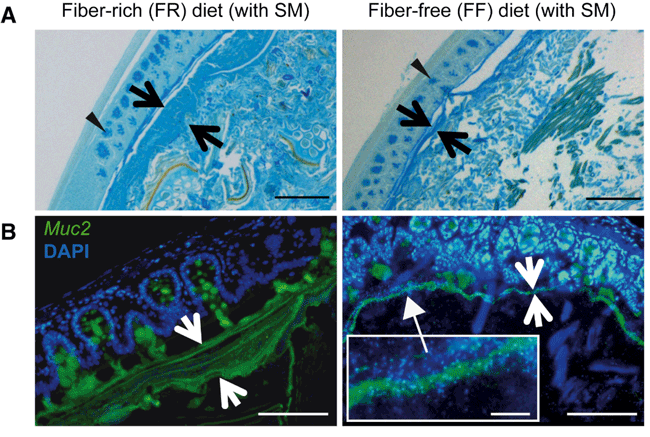
Figure 2. Microbiota-mediated erosion of the colonic mucus barrier and host responses. (A) Alcian blue-stained colonic sections showing the mucus layer (arrows). Scale bars, 100 μm. Opposing black arrows with shafts delineate the mucus layer that was measured and triangular arrowheads point to pre-secretory goblet cells. (B) Immunofluorescence images of colonic thin sections stained with α-Muc2 antibody and DAPI. Opposing white arrows with shafts delineate the mucus layer. Inset (FF diet group) shows a higher magnification of bacteria-sized, DAPI-stained particles in closer proximity to host epithelium and even crossing this barrier. Scale bars: 100 μm; inset, 10 μm. Reprinted from Desai MS, Seekatz AM, et al. A dietary fiber-deprived gut microbiota degrades the colonic mucus barrier and enhances pathogen susceptibility. Cell 167, 5 (2016). https://doi.org/10.1016/j.cell.2016.10.043
When the researchers introduced a pathogen, Citrobacter rodentium, to the fiber-deprived gut, the pathogen had easy access to the gut epithelium and caused lethal colitis.
So far, I’ve focused on the role of gut microbes in preventing gastrointestinal infection. But what about infections outside the gut?
Dietary fiber confers protection against the flu by influencing the formation of new immune cells in the bone marrow
Interestingly, dietary fiber and the resulting SCFAs from bacterial fermentation may also protect against respiratory infection. In 2018, a group of Swiss researchers found that mice fed a high-fiber diet had enhanced antiviral immunity and increased survival from influenza infection.15
Notably, a high-fiber diet also reduced immune-related damage in the lungs. Tissue destruction is common in respiratory infection due to uncontrolled immune responses and can contribute to morbidity and mortality. Here, the researchers found that dietary fiber or butyrate was able to reduce the influx of immune cells called neutrophils into the airways.
This primarily occurred through a gut – bone marrow – lung axis. Gut-derived SCFAs acted on receptors in the bone marrow, where new immune cells are formed. SCFAs specifically increased the number of monocytes that were specialized for tissue protection and repair. These monocytes produced less inflammatory signaling molecules, reducing the recruitment of potentially damaging neutrophils.
Antibiotics reduce immune responses to respiratory virus infection
Given the importance of bacterially derived SCFAs in antiviral responses, it’s not surprising that antibiotics could impair immune responses to infection. A study published in 2011 in the journal PNAS found that treating mice with a cocktail of antibiotics significantly reduced the immune response to influenza virus and increased viral replication in the lungs.
Interestingly, they did not explore altered SCFA signaling as a potential mechanism, and instead found that bacterial-sensing by a specific group of receptors, toll-like receptors (TLRs), in the gut was critical for the response to influenza infection. Mice that had been treated with antibiotics had lower gut levels of lipopolysaccharide (LPS) and other bacterial components, which are potent stimulators of these immune-educating receptors.
Also known as endotoxin for its ability to cross a leaky gut barrier, LPS can get into circulation, where it has been shown to contribute to insulin resistance, systemic inflammation, and depression-like behavior in mice. However, in the gut, LPS is a crucial signal for TLRs, and helps to maintain gut homeostasis.16
PPAR-gamma agonists reduce morbidity and mortality of mice infected with influenza
Another pathway that may be involved is the PPAR-gamma pathway. I’ve written before about how PPAR-gamma acts as the control switch of epithelial metabolism, preventing oxygen leakage into the gut.
Several studies have shown that drugs that are specific activators of the PPAR-gamma pathway, such as rosiglitazone or pioglitazone can reduce morbidity and mortality of mice infected with high pathogenic strains of influenza.17,18 Of course, these drugs are active systemically, so whether gut PPAR-gamma activation is important to this protection is unknown. It would certainly be interesting to see a similar study with mesalamine, a PPAR-gamma agonist with more localized gut activity.
Other research groups have identified dietary compounds with PPAR-gamma dependent activity that can protect against respiratory viral infection and the associated lung tissue damage. These include abscisic acid19, an isoprenoid found in avocado, citrus fruits, and figs, and the flavonoid isoliquiritigenin, found in licorice root.20
Altogether, these studies highlight how highly interconnected the gut is with the rest of our physiology, and how important our gut microbiota is for shaping our immune function. In the next few sections, we’ll review a few other factors that might alter our gut microbes and our resilience to infection.
Stress alters the gut microbiota and exacerbates gut and respiratory infection
Psychological and social stress have long been associated with increased susceptibility to infectious diseases. High levels of cortisol and other stress hormones from continuous activation of the “fight or flight” response can suppress our immune function, both in the gut and elsewhere in the body.
Stress has also been known to alter the composition of the gut microbiota, increasing the relative abundance of pro-inflammatory Proteobacteria at the expense of beneficial butyrate-producing bacteria.21
It can also influence susceptibility to gut infection. When stressed mice were challenged with Citrobacter rodentium, they had greater colonization of the pathogen and more gut inflammation than non-stressed mice.22 Stress has also been shown to increase the infectivity and pathogenicity of Blastocystis, which may be a harmless commensal in lower stress conditions.23
Psychosocial stress can also alter the metabolites that our microbes produce. Research done by my former lab mate, Dr. Jacob Allen, demonstrated that stress reduced the synthesis of B vitamins by our gut microbes and increased markers of systemic inflammation.24 This was somewhat attenuated by the consumption of prebiotic fibers, suggesting that a nutrient-dense diet may somewhat mitigate some of the negative effects of stress.
Proton pump inhibitors increase susceptibility to gut infection
Stomach acid, or gastric acid, is another important factor in the defense against infectious microbes. The normal human stomach has a pH in the range of 1.5 to 3.5. Low stomach acid is referred to as hypochlorhydria. Hypochlorhydria can occur with aging, stress, pernicious anemia, Helicobacter pylori infection, autoimmune gastritis, and hypothyroidism.
However, perhaps the most common cause of hypochlorhydria is the ubiquitous use of proton pump inhibitors. These drugs attempt to control the symptoms of acid reflux by reducing the acidity of the stomach contents, impairing proper digestion and allowing many ingested microbes that would normally be destroyed in the stomach to make their way intact into the gut. Once in the gut, they can begin to compete for an available niche.
Indeed, a recent study found that people who used PPIs had an over-representation of microbes normally found in the oral microbiome.25 Those that use acid-blockers also have a significantly increased chance of being infected with opportunistic bacterial pathogens like C. difficile, Salmonella, Campylobacter, and Shigella.26 PPIs may also increase the risk of gut viral infection with pathogens like norovirus.27
Aging and the gut microbiota: caloric restriction may be protective but is not recommended during infection
Of course, the elderly are particularly susceptible to infection and the associated complications. Aging has often been associated with a general decrease in microbiota diversity and a shift towards a more inflammatory microbiota, with lower abundance of butyrate-producing microbes and a higher abundance of facultative anaerobic Proteobacteria.28
However, if we continue to take care of ourselves into old age, this may not necessarily have to happen. Several studies have shown that healthy, long-lived individuals have a microbiome that looks just as or even more diverse than young individuals.29,30
One strategy that has gained popularity for delaying the aging process is caloric restriction. Caloric restriction has been shown to consistently extend lifespan in animal models and reduce the aging of the immune system. Studies assessing the gut microbiota have found that it promotes a rapid expansion and long-lasting increase of Lactobacillus, a beneficial genus of bacteria with known immune-modulating properties.31
However, a study published in 2005 found that caloric restriction reduced survival of aged mice in response to influenza infection.32 Caloric restriction reduced the activity of natural killer cells in the lungs, leading to increased viral replication. This suggests that, although caloric restriction may positively affect many long-term parameters of aging, acute caloric restriction can increase susceptibility once exposure has occurred.
Another study published in 2017 in the journal Frontiers in Immunology sought to elucidate the role of aging, caloric restriction, and the associated gut microbiota changes on the response to respiratory influenza infection.33 While there was little effect of age, caloric restriction was associated with an increased abundance of pro-inflammatory Proteobacteria in the context of influenza infection. Most interestingly, regardless of age, the relative abundance of Proteobacteria was positively correlated with the severity of infection.
Overall, this suggests that adequate nutrition and abundant calories are essential to supporting gut health and immunity during infection.
Could “re-wilding” the gut microbiota protect against infectious and chronic disease?
Perhaps the most interesting study I came across while researching this article was a 2017 NIH study published in Cell that looked at the effects of “re-wilding” the guts of laboratory mice.46 Much like most modern-day humans, lab mice live in sanitized and restricted environment devoid of natural microbial exposure. As a result, they’ve lost many of the microbes that they co-evolved with.
The researchers first transferred the wild microbiota into laboratory mice over several generations and made sure that it stuck. They then tested the immune response of the “re-wilded” mice to influenza and a carcinogen. Compared to regular lab mice, lab mice reconstituted with the natural microbiota demonstrated reduced inflammation and increased survival after influenza virus infection and improved resistance to developing colorectal cancer.
While there is limited evidence for “re-wilding” the gut in humans, this certainly leads us to wonder whether spending time in natural environments could be important for supporting a healthy gut microbiota, optimizing immunity to infection, and reducing risk of immune-related chronic disease.
Can probiotics protect against respiratory viral infection?
What about probiotics? There are a number of gut bloggers right now promoting probiotic supplements as a way to protect ourselves against respiratory illness. Let’s take a look at the evidence:
A 2015 Cochrane systematic review of on probiotics for preventing acute upper respiratory tract infections (URTI) concluded:
“Probiotics were better than placebo in reducing the number of participants experiencing episodes of acute URTI, the mean duration of an episode of acute URTI, antibiotic use and cold‐related school absence. This indicates that probiotics may be more beneficial than placebo for preventing acute URTIs. However, the quality of the evidence was low or very low.” 34
Of course, whenever we are considering the evidence for probiotics, it is important to consider that the effects are strain- and disease-specific:
- Several randomized controlled trials have shown that Lactobacillus rhamnosus GG* can reduce the risk of upper respiratory infections in young children.35–37 (I recommend Pure Encapsulations, which is free of other ingredients and additives.**) *Note that this strain is not recommended in cases of Crohn’s disease, short bowel syndrome, severe ulcerative colitis, or other cases of severe gut inflammation.
- Lactobacillus acidophilus NCFM may also be effective in reducing fever and cough incidence and duration in a pediatric population.38 (Found in Metagenics Ultra Flora IB**).
- Fewer studies have been done in adult or elderly populations. However, one study found that Bifidobacterium lactis Bl-04 was effective in reducing the risk of URTI in healthy, physically-active adults.39 This strain is difficult to find on its own but is included in the RightBiotics RX formula**, which also contains several other well-studied, soil-based strains (reviewed in my complete guide to soil-based probiotics).
**I have no affiliation with any probiotic companies. Any links to specific brands are simply to preemptively answer reader questions about which brands contain the most well-studied strains.
Overall, taking these specific probiotic strains may provide some benefit, but is not a substitute for good hygiene and general practices to support gut health and overall immunity, which we’ll get to in a moment. First, a quick aside on social distancing and infection itself, and how they could potentially impact gut health.
Could social distancing impact the gut microbiota?
There’s good reason to be social distancing right now. Social contact is a primary way of spreading infectious disease, and flattening the curve is essential for saving lives and preventing burnout among healthcare workers on the front line.
But interestingly, social contact is also a way of sharing harmless or even beneficial microbes with others. Married individuals and those who share living quarters have been shown to end up with gut microbiomes that look more similar to one another.40 Sociability in humans has been associated with higher gut diversity.41
The effects of social ties have been even more extensively studied in primates. Dr. Andrew Moeller and colleagues found that the gut microbes of chimpanzees converged during the seasons when they were most sociable.42 The most sociable individuals, who spent more time grooming, touching, or hanging out with other chimps, had the greatest diversity of species in their guts.
Work done in Kenya on wild baboons showed similar results, even after controlling for diet, shared genetics, and shared environments.43 The authors concluded that these findings “strongly implicate direct physical contact among social partners in the transmission of gut microbial species.”
Can a gut or respiratory infection alter the gut microbiota?
In my recent article on the oxygen-dysbiosis connection, I highlighted how certain gut pathogens can hack gut metabolism and shift the composition of the gut microbiota in their favor. In fact, this may be the underlying pathophysiology present in individuals with post-infectious irritable bowel syndrome. However, the effects of infection on the gut are not limited to gut pathogens.
Respiratory influenza infection in humans is often accompanied by gastroenteritis-like symptoms, including diarrhea. In 2014, a group of researchers in China found that respiratory influenza infection caused significant injury to the small intestine.44 This was not due to intestinal viral infection, but rather due to immune cells that were activated in the lungs traveling to intestinal tissues, causing inflammation.
Respiratory infection decreased the abundance of the beneficial genus Lactobacillus and increased the abundance of pro-inflammatory Proteobacteria.44 In mice, supplemental butyrate has been shown to reduce Proteobacteria expansion in the presence of an enteric pathogen, so it’s plausible that it could similarly be protective in respiratory infection.
Another study published in PLoS One in 2014 found that respiratory Mycobacterium tuberculosis infection resulted in rapid loss of diversity in the gut microbiota as early as six days after lung infection.45 Recovery of gut diversity was fairly rapid, but to a significantly different composition from baseline.
Ways to support your gut-immune axis
Hopefully this article convinced you of the important role that gut health plays in influencing our immune response! Optimizing gut health is always important but taking care of your gut is particularly vital in supporting resilience to infection. Below are a few ways to support the gut-immune axis.
Please note that these general recommendations are based on the principles of supporting overall gut health and the studies of influenza and other well-characterized pathogens reviewed in this article. We currently have very little data to support any specific recommendations for COVID-19, and none of this should be taken as medical advice.
1) Avoid antibiotics whenever possible. Antibiotics provide a window of opportunity for pathogens to take hold, colonize, or expand within the gut. If you have to take antibiotics, see my recommendations in this article for how to support gut recovery, and consider taking extra precautions to minimize exposure to infection during and after antibiotics.
2) Eat a nutrient-dense diet rich in dietary fiber. When we eat dietary fiber, our microbes ferment it into short-chain fatty acids like butyrate that support the integrity of the gut barrier and immune health in the gut and throughout the body.
3) Exercise regularly. Moderate exercise is known to boost immune function, increase our resilience to stress, and support a healthy gut microbiota.
4) Manage stress and get enough sleep. Stress and lack of sleep can significantly impair immune function and increase susceptibility to infection. Stay tuned for an entire future article dedicated to stress and how it impacts the gut.
5) Get time outside in nature. Social distancing is likely reducing our typical exposure to microbes. Getting outside in a natural environment is a great way to increase our microbial exposure and support immune function.
6) Treat any underlying conditions. If you have an underlying condition and are not already actively working to identify and address the root cause of disease, this is a great time to focus on what steps you can take towards better health. For those who may want some additional help in this regard, I still have a few openings for one-on-one consultations.
7) Consider taking probiotics. While significantly less important than optimizing your diet and lifestyle, certain probiotic strains may help to reduce the likelihood of respiratory infection.
8) Consider butyrate supplementation. If you cannot tolerate the prebiotic fiber in whole foods or have a low-abundance of butyrate-producing microbes, this could be a good short-term strategy to support gut health and immune function. See my article series on butyrate for specific recommendations.
That’s all for now! I’d love to hear how you’re doing and what you thought of this article in the comments below.

How gut health affects resistance to infection
The gut microbiome and the immune system are intricately connected, and over 70 percent of the body’s immune cells reside in the gut. Read on to learn how gut health affects our resistance to infection, and how to best support the gut-immune axis.
A lot sure has changed in the last several weeks. There is no doubt that the global response to the COVID-19 pandemic is affecting everyone, whether in small or large ways. I certainly hope that you and yours are safely social distancing at home and taking the appropriate measures to protect yourselves, your community, and our healthcare workers on the front lines.
It’s taken me a while to decide how I want to tackle the topic publicly. I honestly questioned whether it was even my place to tackle it at all. Many physicians, researchers, and organizations that I follow and trust have put out some really amazing resources in regards to how best we can protect our own health and our broader community against COVID-19. At the same time, I’ve seen a lot of misinformation from people in the online health space about this pandemic. I have no pretenses about being an expert in infectious disease and certainly don’t want to contribute to the noise.
Moreover, as much as what is going on now with the COVID-19 pandemic is and should be on everyone’s mind, we are still in the midst of a long-burning epidemic of gut dysbiosis and chronic disease. And given that approximately 90 percent of patients that need to be hospitalized with COVID-19 have one or more underlying conditions (including obesity, hypertension, chronic lung disease, diabetes, and cardiovascular disease), I still see that area as where my knowledge, energy, and content production efforts are best directed.
Nevertheless, it has always been my mission to empower you with the information you need to support your health and live well, so I decided that I would approach this topic more generally, as this is likely not going to be the only time when it might be important to consider how our gut health impacts immunity.
Thus, this article will be a broad overview of what we know and don’t know about how our gut health influences resistance to infection. I will start with just a few brief notes on the transmission and presentation of COVID-19 that will hopefully provide context for the remainder of the article.
If you’re looking for more detailed evidence-based information about COVID-19, I recommend checking out the videos by MedCram or Ninja Nerd Science and the research updates provided by the New England Journal of Medicine in addition to keeping up to date with WHO, CDC, and local recommendations.
On COVID-19 and the gut
- While the novel coronavirus, SARS-CoV-2, seems to be principally spread by respiratory droplets, the fecal-oral route is also a potential route of transmission.1,2 This means that the live virus may be shed through fecal material and spread to the mouth or other mucosal surfaces through unwashed hands.
- Entry of the virus into the body requires both the cell receptor ACE2 and a transmembrane protein called TMPRSS2.3 These proteins are not only present in the type 2 lung alveolar cells but are also highly expressed in absorptive cells of the small intestine and colon.4
- Many people present with digestive symptoms. According to a recent report, 50.5 percent of individuals admitted to the hospital for COVID-19 in Hubei, China presented with digestive symptoms, including diarrhea, vomiting, and abdominal pain.5 For many patients, digestive symptoms preceded the development of cough, shortness of breath, or fever. A small percentage may present with digestive symptoms alone.
- The virus can be shed in stool, even for as long as five weeks after respiratory symptoms have resolved.6
- COVID-19 has been reported to cause gut inflammation.7 Whether it is capable of producing any long-term damage to the gut, as it seems to be able to do in the lungs, is still unknown.
The gut barrier: first line of defense
To start, I’m going to review the basics of the gut barrier and gut immune system. In many ways, we can think of the interior of the gut as being outside of the body. Much like the hole of a doughnut is not actually part of the doughnut, the gut is really just a hollow tube that connects the mouth and rectum.
The gut barrier is composed of a single layer of specialized epithelial cells that are about the width of a human hair. These epithelial cells must form a tight barrier to prevent microbes, microbial components, and large dietary proteins from entering the body.
To maintain a tight barrier, gut epithelial cells secrete a wide range of antimicrobial peptides, which repel microbes from the epithelium, and a thick, protective layer of mucus that sits on top of the epithelial layer. Protein complexes called tight junctions connect adjacent epithelial cells, preventing anything from slipping through the cracks between epithelial cells.
If the gut mucus layer or gut barrier breaks down, this is called intestinal permeability, or “leaky gut”. This intestinal permeability gives microbes easier access to the body and can lead to chronic inflammation or infection if these microbes overwhelm the gut immune system.
The gut-immune system: 101
An estimated 70 percent of the immune cells in our bodies reside in the gut. These immune cells play a crucial role in determining which microbes are able to colonize and survive in the gut.
Our gut microbes, in turn, are constantly communicating to our gut immune cells. These immune cells lie just beneath the gut barrier in a region called the gut-associated lymphoid tissue, or GALT.

Figure 1. Architecture of the gut-associated lymphoid tissue. Reprinted from Brucklacher-Waldert et al. Cellular plasticity of CD4+ T cells in the intestine. Frontiers in Immunology (2014). https://doi.org/10.3389/fimmu.2014.00488
Early in life, the GALT undergoes a maturation process as the gut immune system learns to distinguish harmless host cells, dietary proteins, and commensal bacteria from pathogenic microbes and environmental toxins. This education of our immune system is essential for its proper functioning. In fact, mice that are raised in a sterile environment (germ-free mice) and do not have a gut microbiota have a severely underdeveloped gut immune system.8
Throughout life, gut microbes continue to shape and regulate the immune system. Gut immune cells have specialized receptors that sense the presence of microbes. This microbe-sensing is critical to maintaining gut homeostasis and gut barrier function.
Alright – now that we’ve reviewed the basics, lets get to the more interesting research!
A healthy gut keeps out potential invaders through colonization resistance
In a recent article, I discussed the concept of nutrient-niches within the gut.
A niche is a multidimensional space of resources and environmental conditions that define where an organism can survive and grow.
In the gut, available niches are primarily determined by the nutrients that comprise the host diet, hence the name nutrient-niches.9
When our gut microbiota is intact, all of the nutrient-niches are completely filled by microbes. This means that any new or harmful microbe will find it difficult to find a niche within which it can colonize and survive in the gut. This is a concept called colonization resistance.10
If our gut undergoes a significant disturbance, however, it will no longer resist invasion by new microbes. Antibiotics, for instance, can “unseat” microbes from their nutrient-niches, allowing new or existing pathogens the opportunity to take hold and cause infection in the gut.
As we’ll see in the next few sections, a poor diet, stress, and other environmental factors can also alter nutrient-niches and reduce our resistance to gut infection.
Dietary fiber supports the gut barrier and reduces susceptibility to infection
When we consume dietary fiber, our gut microbes ferment it into short-chain fatty acids (SCFAs). These SCFAs provide energy for the cells that form the gut barrier, and also stimulate the secretion of a protective layer of mucus that keeps microbes a safe distance from our gut epithelium. The three most abundant short-chain fatty acids in the gut are butyrate, acetate, and propionate.
In 2018, a group of Stanford researchers found that bacteria in the genus Bacteroides produce the SCFA propionate, which limits the growth and colonization of Salmonella.11 Similarly, depletion of butyrate-producing Clostridia has been shown to allow for expansion of Salmonella in the mouse gut.12 Butyrate has also been shown to increase the resistance of intestinal epithelial cells to toxins produced by C. difficile through supporting gut hypoxia.13
Consuming fiber may be essential to producing enough of these SCFAs to effectively repel incoming pathogens. Work done by Dr. Eric Martens’ lab at the University of Michigan found that in mice, depriving the gut of dietary fiber results in a significant expansion of microbes like Akkermansia muciniphila and Bacteroides caccae, which are able to feed on host mucus glycans.14 While these microbes have been shown to be beneficial at low abundance, their rapid expansion and increased activity degraded the protective gut mucus layer.
Figure 2. Microbiota-mediated erosion of the colonic mucus barrier and host responses. (A) Alcian blue-stained colonic sections showing the mucus layer (arrows). Scale bars, 100 μm. Opposing black arrows with shafts delineate the mucus layer that was measured and triangular arrowheads point to pre-secretory goblet cells. (B) Immunofluorescence images of colonic thin sections stained with α-Muc2 antibody and DAPI. Opposing white arrows with shafts delineate the mucus layer. Inset (FF diet group) shows a higher magnification of bacteria-sized, DAPI-stained particles in closer proximity to host epithelium and even crossing this barrier. Scale bars: 100 μm; inset, 10 μm. Reprinted from Desai MS, Seekatz AM, et al. A dietary fiber-deprived gut microbiota degrades the colonic mucus barrier and enhances pathogen susceptibility. Cell 167, 5 (2016). https://doi.org/10.1016/j.cell.2016.10.043
When the researchers introduced a pathogen, Citrobacter rodentium, to the fiber-deprived gut, the pathogen had easy access to the gut epithelium and caused lethal colitis.
So far, I’ve focused on the role of gut microbes in preventing gastrointestinal infection. But what about infections outside the gut?
Dietary fiber confers protection against the flu by influencing the formation of new immune cells in the bone marrow
Interestingly, dietary fiber and the resulting SCFAs from bacterial fermentation may also protect against respiratory infection. In 2018, a group of Swiss researchers found that mice fed a high-fiber diet had enhanced antiviral immunity and increased survival from influenza infection.15
Notably, a high-fiber diet also reduced immune-related damage in the lungs. Tissue destruction is common in respiratory infection due to uncontrolled immune responses and can contribute to morbidity and mortality. Here, the researchers found that dietary fiber or butyrate was able to reduce the influx of immune cells called neutrophils into the airways.
This primarily occurred through a gut – bone marrow – lung axis. Gut-derived SCFAs acted on receptors in the bone marrow, where new immune cells are formed. SCFAs specifically increased the number of monocytes that were specialized for tissue protection and repair. These monocytes produced less inflammatory signaling molecules, reducing the recruitment of potentially damaging neutrophils.
Antibiotics reduce immune responses to respiratory virus infection
Given the importance of bacterially derived SCFAs in antiviral responses, it’s not surprising that antibiotics could impair immune responses to infection. A study published in 2011 in the journal PNAS found that treating mice with a cocktail of antibiotics significantly reduced the immune response to influenza virus and increased viral replication in the lungs.
Interestingly, they did not explore altered SCFA signaling as a potential mechanism, and instead found that bacterial-sensing by a specific group of receptors, toll-like receptors (TLRs), in the gut was critical for the response to influenza infection. Mice that had been treated with antibiotics had lower gut levels of lipopolysaccharide (LPS) and other bacterial components, which are potent stimulators of these immune-educating receptors.
Also known as endotoxin for its ability to cross a leaky gut barrier, LPS can get into circulation, where it has been shown to contribute to insulin resistance, systemic inflammation, and depression-like behavior in mice. However, in the gut, LPS is a crucial signal for TLRs, and helps to maintain gut homeostasis.16
PPAR-gamma agonists reduce morbidity and mortality of mice infected with influenza
Another pathway that may be involved is the PPAR-gamma pathway. I’ve written before about how PPAR-gamma acts as the control switch of epithelial metabolism, preventing oxygen leakage into the gut.
Several studies have shown that drugs that are specific activators of the PPAR-gamma pathway, such as rosiglitazone or pioglitazone can reduce morbidity and mortality of mice infected with high pathogenic strains of influenza.17,18 Of course, these drugs are active systemically, so whether gut PPAR-gamma activation is important to this protection is unknown. It would certainly be interesting to see a similar study with mesalamine, a PPAR-gamma agonist with more localized gut activity.
Other research groups have identified dietary compounds with PPAR-gamma dependent activity that can protect against respiratory viral infection and the associated lung tissue damage. These include abscisic acid19, an isoprenoid found in avocado, citrus fruits, and figs, and the flavonoid isoliquiritigenin, found in licorice root.20
Altogether, these studies highlight how highly interconnected the gut is with the rest of our physiology, and how important our gut microbiota is for shaping our immune function. In the next few sections, we’ll review a few other factors that might alter our gut microbes and our resilience to infection.
Stress alters the gut microbiota and exacerbates gut and respiratory infection
Psychological and social stress have long been associated with increased susceptibility to infectious diseases. High levels of cortisol and other stress hormones from continuous activation of the “fight or flight” response can suppress our immune function, both in the gut and elsewhere in the body.
Stress has also been known to alter the composition of the gut microbiota, increasing the relative abundance of pro-inflammatory Proteobacteria at the expense of beneficial butyrate-producing bacteria.21
It can also influence susceptibility to gut infection. When stressed mice were challenged with Citrobacter rodentium, they had greater colonization of the pathogen and more gut inflammation than non-stressed mice.22 Stress has also been shown to increase the infectivity and pathogenicity of Blastocystis, which may be a harmless commensal in lower stress conditions.23
Psychosocial stress can also alter the metabolites that our microbes produce. Research done by my former lab mate, Dr. Jacob Allen, demonstrated that stress reduced the synthesis of B vitamins by our gut microbes and increased markers of systemic inflammation.24 This was somewhat attenuated by the consumption of prebiotic fibers, suggesting that a nutrient-dense diet may somewhat mitigate some of the negative effects of stress.
Proton pump inhibitors increase susceptibility to gut infection
Stomach acid, or gastric acid, is another important factor in the defense against infectious microbes. The normal human stomach has a pH in the range of 1.5 to 3.5. Low stomach acid is referred to as hypochlorhydria. Hypochlorhydria can occur with aging, stress, pernicious anemia, Helicobacter pylori infection, autoimmune gastritis, and hypothyroidism.
However, perhaps the most common cause of hypochlorhydria is the ubiquitous use of proton pump inhibitors. These drugs attempt to control the symptoms of acid reflux by reducing the acidity of the stomach contents, impairing proper digestion and allowing many ingested microbes that would normally be destroyed in the stomach to make their way intact into the gut. Once in the gut, they can begin to compete for an available niche.
Indeed, a recent study found that people who used PPIs had an over-representation of microbes normally found in the oral microbiome.25 Those that use acid-blockers also have a significantly increased chance of being infected with opportunistic bacterial pathogens like C. difficile, Salmonella, Campylobacter, and Shigella.26 PPIs may also increase the risk of gut viral infection with pathogens like norovirus.27
Aging and the gut microbiota: caloric restriction may be protective but is not recommended during infection
Of course, the elderly are particularly susceptible to infection and the associated complications. Aging has often been associated with a general decrease in microbiota diversity and a shift towards a more inflammatory microbiota, with lower abundance of butyrate-producing microbes and a higher abundance of facultative anaerobic Proteobacteria.28
However, if we continue to take care of ourselves into old age, this may not necessarily have to happen. Several studies have shown that healthy, long-lived individuals have a microbiome that looks just as or even more diverse than young individuals.29,30
One strategy that has gained popularity for delaying the aging process is caloric restriction. Caloric restriction has been shown to consistently extend lifespan in animal models and reduce the aging of the immune system. Studies assessing the gut microbiota have found that it promotes a rapid expansion and long-lasting increase of Lactobacillus, a beneficial genus of bacteria with known immune-modulating properties.31
However, a study published in 2005 found that caloric restriction reduced survival of aged mice in response to influenza infection.32 Caloric restriction reduced the activity of natural killer cells in the lungs, leading to increased viral replication. This suggests that, although caloric restriction may positively affect many long-term parameters of aging, acute caloric restriction can increase susceptibility once exposure has occurred.
Another study published in 2017 in the journal Frontiers in Immunology sought to elucidate the role of aging, caloric restriction, and the associated gut microbiota changes on the response to respiratory influenza infection.33 While there was little effect of age, caloric restriction was associated with an increased abundance of pro-inflammatory Proteobacteria in the context of influenza infection. Most interestingly, regardless of age, the relative abundance of Proteobacteria was positively correlated with the severity of infection.
Overall, this suggests that adequate nutrition and abundant calories are essential to supporting gut health and immunity during infection.
Could “re-wilding” the gut microbiota protect against infectious and chronic disease?
Perhaps the most interesting study I came across while researching this article was a 2017 NIH study published in Cell that looked at the effects of “re-wilding” the guts of laboratory mice.46 Much like most modern-day humans, lab mice live in sanitized and restricted environment devoid of natural microbial exposure. As a result, they’ve lost many of the microbes that they co-evolved with.
The researchers first transferred the wild microbiota into laboratory mice over several generations and made sure that it stuck. They then tested the immune response of the “re-wilded” mice to influenza and a carcinogen. Compared to regular lab mice, lab mice reconstituted with the natural microbiota demonstrated reduced inflammation and increased survival after influenza virus infection and improved resistance to developing colorectal cancer.
While there is limited evidence for “re-wilding” the gut in humans, this certainly leads us to wonder whether spending time in natural environments could be important for supporting a healthy gut microbiota, optimizing immunity to infection, and reducing risk of immune-related chronic disease.
Can probiotics protect against respiratory viral infection?
What about probiotics? There are a number of gut bloggers right now promoting probiotic supplements as a way to protect ourselves against respiratory illness. Let’s take a look at the evidence:
A 2015 Cochrane systematic review of on probiotics for preventing acute upper respiratory tract infections (URTI) concluded:
“Probiotics were better than placebo in reducing the number of participants experiencing episodes of acute URTI, the mean duration of an episode of acute URTI, antibiotic use and cold‐related school absence. This indicates that probiotics may be more beneficial than placebo for preventing acute URTIs. However, the quality of the evidence was low or very low.” 34
Of course, whenever we are considering the evidence for probiotics, it is important to consider that the effects are strain- and disease-specific:
- Several randomized controlled trials have shown that Lactobacillus rhamnosus GG* can reduce the risk of upper respiratory infections in young children.35–37 (I recommend Pure Encapsulations, which is free of other ingredients and additives.**) *Note that this strain is not recommended in cases of Crohn’s disease, short bowel syndrome, severe ulcerative colitis, or other cases of severe gut inflammation.
- Lactobacillus acidophilus NCFM may also be effective in reducing fever and cough incidence and duration in a pediatric population.38 (Found in Metagenics Ultra Flora IB**).
- Fewer studies have been done in adult or elderly populations. However, one study found that Bifidobacterium lactis Bl-04 was effective in reducing the risk of URTI in healthy, physically-active adults.39 This strain is difficult to find on its own but is included in the RightBiotics RX formula**, which also contains several other well-studied, soil-based strains (reviewed in my complete guide to soil-based probiotics).
**I have no affiliation with any probiotic companies. Any links to specific brands are simply to preemptively answer reader questions about which brands contain the most well-studied strains.
Overall, taking these specific probiotic strains may provide some benefit, but is not a substitute for good hygiene and general practices to support gut health and overall immunity, which we’ll get to in a moment. First, a quick aside on social distancing and infection itself, and how they could potentially impact gut health.
Could social distancing impact the gut microbiota?
There’s good reason to be social distancing right now. Social contact is a primary way of spreading infectious disease, and flattening the curve is essential for saving lives and preventing burnout among healthcare workers on the front line.
But interestingly, social contact is also a way of sharing harmless or even beneficial microbes with others. Married individuals and those who share living quarters have been shown to end up with gut microbiomes that look more similar to one another.40 Sociability in humans has been associated with higher gut diversity.41
The effects of social ties have been even more extensively studied in primates. Dr. Andrew Moeller and colleagues found that the gut microbes of chimpanzees converged during the seasons when they were most sociable.42 The most sociable individuals, who spent more time grooming, touching, or hanging out with other chimps, had the greatest diversity of species in their guts.
Work done in Kenya on wild baboons showed similar results, even after controlling for diet, shared genetics, and shared environments.43 The authors concluded that these findings “strongly implicate direct physical contact among social partners in the transmission of gut microbial species.”
Can a gut or respiratory infection alter the gut microbiota?
In my recent article on the oxygen-dysbiosis connection, I highlighted how certain gut pathogens can hack gut metabolism and shift the composition of the gut microbiota in their favor. In fact, this may be the underlying pathophysiology present in individuals with post-infectious irritable bowel syndrome. However, the effects of infection on the gut are not limited to gut pathogens.
Respiratory influenza infection in humans is often accompanied by gastroenteritis-like symptoms, including diarrhea. In 2014, a group of researchers in China found that respiratory influenza infection caused significant injury to the small intestine.44 This was not due to intestinal viral infection, but rather due to immune cells that were activated in the lungs traveling to intestinal tissues, causing inflammation.
Respiratory infection decreased the abundance of the beneficial genus Lactobacillus and increased the abundance of pro-inflammatory Proteobacteria.44 In mice, supplemental butyrate has been shown to reduce Proteobacteria expansion in the presence of an enteric pathogen, so it’s plausible that it could similarly be protective in respiratory infection.
Another study published in PLoS One in 2014 found that respiratory Mycobacterium tuberculosis infection resulted in rapid loss of diversity in the gut microbiota as early as six days after lung infection.45 Recovery of gut diversity was fairly rapid, but to a significantly different composition from baseline.
Ways to support your gut-immune axis
Hopefully this article convinced you of the important role that gut health plays in influencing our immune response! Optimizing gut health is always important but taking care of your gut is particularly vital in supporting resilience to infection. Below are a few ways to support the gut-immune axis.
Please note that these general recommendations are based on the principles of supporting overall gut health and the studies of influenza and other well-characterized pathogens reviewed in this article. We currently have very little data to support any specific recommendations for COVID-19, and none of this should be taken as medical advice.
1) Avoid antibiotics whenever possible. Antibiotics provide a window of opportunity for pathogens to take hold, colonize, or expand within the gut. If you have to take antibiotics, see my recommendations in this article for how to support gut recovery, and consider taking extra precautions to minimize exposure to infection during and after antibiotics.
2) Eat a nutrient-dense diet rich in dietary fiber. When we eat dietary fiber, our microbes ferment it into short-chain fatty acids like butyrate that support the integrity of the gut barrier and immune health in the gut and throughout the body.
3) Exercise regularly. Moderate exercise is known to boost immune function, increase our resilience to stress, and support a healthy gut microbiota.
4) Manage stress and get enough sleep. Stress and lack of sleep can significantly impair immune function and increase susceptibility to infection. Stay tuned for an entire future article dedicated to stress and how it impacts the gut.
5) Get time outside in nature. Social distancing is likely reducing our typical exposure to microbes. Getting outside in a natural environment is a great way to increase our microbial exposure and support immune function.
6) Treat any underlying conditions. If you have an underlying condition and are not already actively working to identify and address the root cause of disease, this is a great time to focus on what steps you can take towards better health. For those who may want some additional help in this regard, I still have a few openings for one-on-one consultations.
7) Consider taking probiotics. While significantly less important than optimizing your diet and lifestyle, certain probiotic strains may help to reduce the likelihood of respiratory infection.
8) Consider butyrate supplementation. If you cannot tolerate the prebiotic fiber in whole foods or have a low-abundance of butyrate-producing microbes, this could be a good short-term strategy to support gut health and immune function. See my article series on butyrate for specific recommendations.
That’s all for now! I’d love to hear how you’re doing and what you thought of this article in the comments below.



Lucy–to what degree can someone go from a dysbiosis to a really robust and healthy biome that can fight infection? How long does it take? How would you know if you were getting somewhere? Thanks!
[…] recently wrote a broad overview on what we know – and what we don’t know – about the role of the gut in the immune […]
This article was so very informative! Thank you, Lucy!
I’ve been mostly carnivore for 18 months now and fiber has rarely passed through my teeth during this time. My digestive system has been more at peace than any other time in my life (64 now) and I have not had a cold/flu during this time. Had bloodwork in January and inflammatory markers were in the “very low risk” range. Of course, this makes me wonder about the emphasis on fiber. Also, my cholesterol is very high, which I’ve read is now being associated with stronger immune function. So, the fiber thing has me confused. Any help here?
There is no information about impact of intermittent fasting on repairing intestinal permeability.